|

 Biochemical Pharmacology
Biochemical Pharmacology
ELSEVIER
Biochemical Phannaco1ogy 7194
(2002) 1-7
The alkaloid sanguinarine is effective
against multi drug resistance in human cervical cells
via bimodal cell death
Zhihu Ding (a), Shou-Ching
Tang (a,b), Priya Weerasinghe (a),
Xiaolong Yang (a), Alan
Pater(a,*), Andrejs Liepins (a)
a -"Division of Basic Sciences,
Faculty of Medicine, Memorial University of
Newfoundland, 300 Prince Philip Drive, St. Johns, NF,
Canada AiB 3V6
b - Newfoundland Cancer Treatment and Research
Foundation, DI: H. Bliss Murphy Cancer Cente/;
300 Prince Philip Drive, St. Johns, NF, Canada AiB
3V6
Received
1 June 2001; accepted 12 October 2001
Abstract
Sanguinarine, a benzophenanthrine alkaloid,
is potentially antineoplastic through induction of cell
death pathways. The development of multidrug resistance
(MDR) is a major obstacle to the success of
chemotherapeutic agents. The aim of this study was to
investigate whether sanguinarine is effective against
uterine cervical MDR and, if so, by which mechanism.
The effects of treatment with sanguinarine on human
papillomavirus (HPV) type 16-immortalized endocervical
cells and their MDR counterpart cells were compared.
Trypan blue exclusion assays and clonogenic survival
assays demonstrated that MDR human cervical cells are
as sensitive as their drug-sensitive parental cells to
death induced by sanguinarine. Upon treatment of both
types of cells with sanguinarine, two distinct
concentration-dependent modes of cell death were
observed. Treatment with 2.12 or 4.24 uM
sanguinarine induced death in most cells that was
characterized as apoptosis using the criteria of cell
surface blebbing, as determined by light and scanning
electron microscopy, and proteolytic activation of
caspase-3 and cleavage of the caspase-3 substrate
poly(ADP-ribose) polymerase (PARP), as detected by
western blot analysis. However, 8.48 and 16.96
uM sanguinarine caused a second mode of cell
death, oncosis, distinguished by cell surface
blistering, and neither caspase-3 activation nor PARP
cleavage. This study provides the first evidence that
sanguinarine is effective against MDR in cervical cells
via bimodal cell death, which displays
alternative mechanisms involving different morphologies
and caspase-3 activation status. C 2002 Published by
Elsevier Science Inc.
Keywords: Alkaloid sanguinarine;
Multidrug-resistant cervical cells; Apoptosis;
Oncosis;Caspase-3
1. Introduction
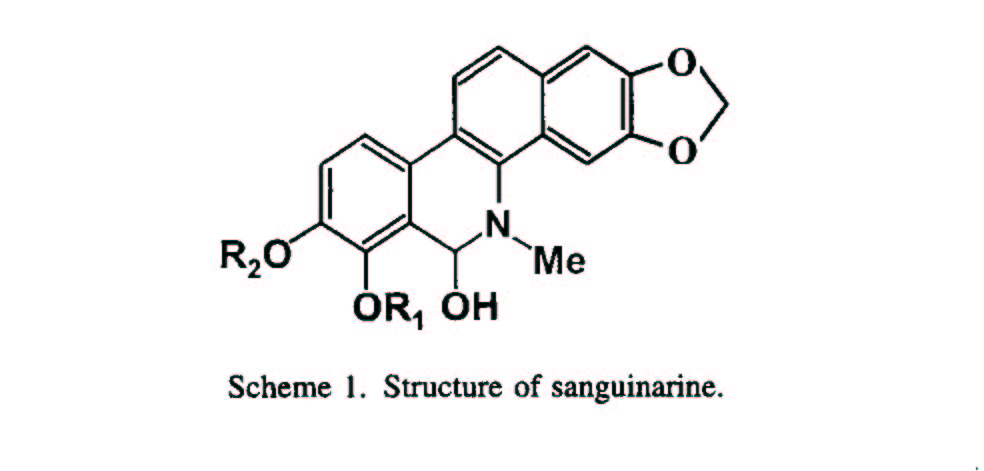
Sanguinarine (Scheme
1) is derived from the plant Sanguinaria
canadensis [1]. Its principal pharmacologic use to
date is in dental products based on its antibacterial,
antifungal, and anti-inflammatory activities, which
reduce gingival inflammation and supragingival plaque
formation [2-4]. Sanguinarine also has been reported to
have antiviral and tumor-targeting activity [5-7].
Molecular biological studies indicate that
sanguinarine has multiple cellular targets [8]. For
example, it can interact with and intercalate DNA
[9,10], inhibit micro- tubule assembly [11], affect
membrane permeability [12,13], and inhibit a wide
variety of enzymes, including Na+/K+ ATPase [14]. Most
interestingly, it also is a potent inhibitor of protein
kinases [15] and NF-KB [16], which are involved in
signal transduction pathways leading to cell
proliferation and/or cell death [7].
Cell death is important for normal
homeostasis, cell proliferation, and differentiation.
The importance of cell death is demonstrated by the
observation that dysregulation of cell death can lead
to cancer, developmental abnormalities, and autoimmune
disorders [17-19]. Cells undergoing PCD (or apoptosis)
are characterized by morphologic changes, including
cellular shrinkage, blebbing, and nuclear DNA
condensation with or without fragmentation [20-24].
However, it is stated that apoptosis is rarely observed
in vivo and may not be the sole mechanism of
cell death [25]. The discovery of intact novel forms of
cell death pathways induced by potential anticancer
agents may have an important bearing in overcoming
chemoresistance.
Of all neoplasms found in females
worldwide, cervical cancer has the third highest
incidence and is fourth on the list of the leading
causes of death by cancer [26,27]. The available drugs
most commonly used for treating cervical malignancies
are impeded by frequent progression to chemotherapy
resistance. Sanguinarine may be effective against MDR,
since the related Sanguinaria canadensis-derived
alkaloid, chelerythrine, has been shown to be cytotoxic
to cancer cells and MDR cells [28]. In this study, we
used our recently established in vitro cervical
cancer model system for MDR [29] to investigate whether
sanguinarine is effective against MDR in human cervical
cells, and to understand the cellular and molecular
mechanisms by which it may induce cell death.
2. Materials and methods
2.1. Cell culture, cell viability
assays, and clonogenic survival assays
Most cell culture protocols,
the HPV type 16-immortalized human endocervical cell
line (HEN-16-2), the CSC- transformed HEN-16-2 cell
line (HEN-16-2T), and the MDR HEN-16-2 cell line
(HEN-16-2/CDDP) have been described previously [29-31].
Cells were cultured in keratinocyte growth medium
(KGM). HeLa cervical carcinoma, CEM- VLB leukemia, CEM-
T4 leukemia, K562 erythroleukemia, and 1M1 pre-B cell
lymphoblastic cells were obtained from the American
Type Culture Collection. HeLa cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal bovine serum (FBS). CEM- VLB leukemia, CEM-
T4 leukemia, K562 erythroleukemia, and 1M1 pre-B cell
lymphoblastic cells were cultured in RPMI-1640 medium
supplemented with 10% FBS. All experiments were
performed in triplicate.
Sanguinarine chloride (Sigma) was dissolved
in H2O as a 2.72 M stock solution, aliquots of which
were serially diluted with KGM and used when needed to
prepare fresh working solutions.
To examine the effect of sanguinarine on
cell viability, 5 x 104 cells/well were seeded in
12-well plates, incubated for 4 or 48 hr, treated with
various concentrations of sanguinarine, and then
assayed for trypan blue exclusion and propidium iodide
exclusion under light microscopy, as described
previously [32,33]. A hemocytometer was used to count
the cells.
Clonogenic survival assays were performed
to examine the combined survival and proliferative
potential of sanguinarine-treated cervical cells, as
previously described [29,34]. Briefly, 103 cells were
seeded int060-mm plates, incubated with 0-16.96
uM sanguinarine for 24 hr, washed twice with
phosphate-buffered saline, and incubated without
sanguinarine for 10-14 days. The cells were stained
with 2% (w/v) crystal violet in methanol, and colonies
of 50 or more cells were counted using a
hemocytometer.
2.2. Cell morphology
analysis
To examine the effect of
sanguinarine on cell morphology under light microscopy,
5 x 103 cells/chamber were seeded in 8-chamber slides
(Nalge Nunc International), incubated for 24 hr, and
treated with 0-16.96 J.lM sanguinarine for 4 hr.
To examine the cell ultrastructural effect
of sanguinarine under scanning electron microscopy
(SEM), 5 x 104 cells/ well were seeded in 12-well
plates containing acid-cleaned coverslips (Lux
Scientific Corp.), incubated for 24 hr for attachment
to coverslips, treated with 0-16.96 uM
sanguinarine for 4 hr, fixed in Karnovsky fixative
containing 2.5% (v/v) glutaraldehyde (J.B. EM Services)
in 0.1 M sodium cacodylate buffer, and then dehydrated
in a 25, 50, 75, and 100% (v/v) ethanol series followed
by Freon 113 substitution. All samples were dried
simultaneously, sputter-coated with gold, and examined
under a Hitachi S-570 scanning electron microscope, as
previously described [35].
2.3. Western blot analysis
Western blot analysis of the
effect of sanguinarine on caspase-3 activation and PARP
cleavage was performed as described previously [36].
Briefly, 10 J.1g of protein was resolved by 10%
(w/v) SDS-PAGE and transferred to Hybond
enhanced chemiluminescence (ECL) nitrocellu- lose
membrane under semidry conditions. Irnrnunodetec- tion
was performed using the ECL system (Amersham Pharmacia
Biotech). Procaspase-3 and caspase-3 were probed using
anti-caspase-3 monoclonal antibody (mAb) (Santa Cruz
Biotechnology). Full-length and cleaved frag- ments
ofPARP were probed usinganti-PARP mAb (Phar-
Mingen).
3. Results
3.1. Evasion of MDR of human
cervical HEN-16-2/ CDDP cells by
sanguinarine
Table I
Bimodal cell death characteristics induced by
sanguinarine and Ukrain in cervical cells and leukemia
cells

HEN-16-2a
PCD/+/+
BCD/-/-
PCD/+/+
BCD/-/-
HEN-16-2/CDDpb
PCD/+/+
BCD/-/-
PCD/+/+
BCD/-/-
HEN-16-2T"
PCD/+/+
BCD/-/-
PCD/+/+
BCD/-/-
HeLad
PCD/+/+
BCD/-/-
PCD/+/+
BCD/-/-
CEM-
T4.
PCD/NA/NA
BCD/NA/NA
NA/NA/NA
NA/NA/NA
CEM-VLBf
PCD/NA/NA
BCD/NA/NA
NA/NA/NA
NA/NA/NA
JMlg
PCD/NA/NA
BCD/NA/NA
PCD/NA/NA
BCD/NA/NA
K562h
PCD/NA/NA
BCD/NA/NA
PCD/NA/NA
BCD/NA/NA
Cells were treated with a dilution
series of sanguinarine or Ukrain for 4 hr and
morphologic changes were observed by microscopy. For
examining caspase-3 activation and PARP cleavage, cell
lysates were subjected to western blotting using
anti-caspase-3 mAb and anti-PARP mAb. PCD programmed
cell death or apoptosis; BCD, blister cell death or
oncosis; NA, not available.
a Human endocervical (HEN)
immortalized with HPVI6.
b HEN-l 6-2 transformed by cisplatin
and MDR.
c HEN-16-2 transformed by cigarette
smoke condensate.
d Endocervical carcinoma.
e Leukemia P-gp-negative.
f Leukemia P-gp-positive.
g Pre-B cell lymphoblastic cell line,
Bcl-2 high level.
h Erythroleukemia cell line, Bcl-210w
level.
We examined the
chemotherapeutic potential of sanguinarine for
MDR cervical cancer cells in a human cervical
in vitro system, which is composed of MDR
HEN-I6-2/ CDDP cells and their drug-sensitive parental
HEN-I6-2 cells [29]. Cell viability, measured by the
trypan blue exclusion assay, was similar in both types
of cells treated with 0, 0.13, 0.26, 0.53, 1.06,2.12,
and 4.24 uM sanguinarine for 4 or 48 hr (Table
1; Fig. 1). The propidium iodide exclusion assay also
showed no significant difference in cell viability
between MDR HEN-16-2/CDDP cells and their
parental HEN-16-2 cells after treatment with these
concentrations of sanguinarine for 4 or 48 hr (data not
shown). Treating the MDR HEN-16-2/CDDP and HEN-
16-2 cells with 0-1.06 uM sanguinarine produced
no significant increase in cell viability; however,
2.12 uM (Fig. 2) and 4.24 uM sanguinarine
treatment caused the death of most of the cells (data
not shown). Treating HEN-16-2/ CDDP and HEN-16-2 cells
with 8.48 and 16.96 uM sanguinarine resulted in
100% cell death within 48 hr (data not shown).
Clonogenic survival assays (also called colony-forming
assays) revealed no significant difference in
clonogenic survival between HEN-16-2/CDDP and HEN-16- 2
cells (data not shown), showing an equally effective
potential of sanguinarine in this assay to kill cells
and inhibit their growth.
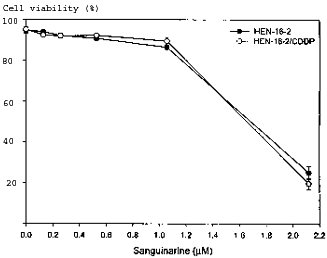
Fig. I. Concentration-dependent effect
of sanguinarine on HEN-16-2 and HEN-16-2/CDDP cell
viability. Cells were incubated with 0, 0.13, 0.26,
0.53, 1.06, and 2.12 uM sanguinarine for 48 hr. Cell
viability represents the percentage of treated compared
with untreated cells that excluded trypan blue dye. The
results represent the means +/- SD of three independent
experiments.
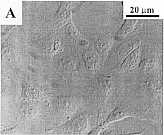 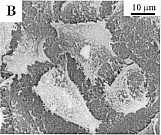
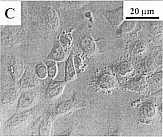 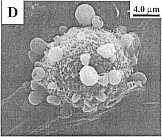
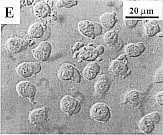 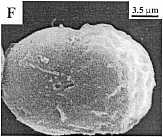
Fig. 2. Concentration-dependent bimodal
effect of sanguinarine on the morphology of MDR
HEN-16-2/CDDP cells. The panels represent untreated
control cells under light microscopy (A) and scanning
electron microscopy (SEM) (B); 4 hr, 2.12 uM
sanguinarine-treated cells under light microscopy (C)
and SEM (D); and 4 hr, 8.48 uM
sanguinarine-treated cells under light microscopy (E)
and SEM (F).
3.2. Induction of
concentration-dependent apoptosis and oncosis in MDR
HEN-I6-2/CDDP and drug-sensitive HEN-I6-2 cervical
cells by sanguinarine
To evaluate the
concentration-dependent effect of sanguinarine on cell
death morphology, cells were treated with different
concentrations of sanguinarine for 4 hr and observed
microscopically. Both cell lines treated with 0- 1.06
uM sanguinarine were observed to have normal
cell morphology, similar to the morphology of untreated
MDR cells (Table 1; Fig. 2A and B). Cell plasma
membrane blebbing, a characteristic of PCD or
apoptosis, was observed in cells treated for 4 hr with
2.12 uM sanguinarine (Fig. 2C and D) and 4.24
uM sanguinarine (Table I). However, most cells
exhibited single and rare double cell surface blisters
after sanguinarine treatment for 4 hr with 8.48
uM (Fig. 2E and F) and 16.96 uM (Table
I). Similar bimodal apoptosis and BCD (or oncosis) were
observed at the same respective sanguinarine
concentrations and time in CSC-transformed HEN-16-2T,
HeLacells, MDR CEM-VLB leukemia, drug-sensitive CEM-T4
leukemia, K562 erythroleukemia, and JM1 pre-B cell
lymphoblastic cells (Table I).
(A)
32kDa-1
______________ 0.5 hr
__________ __________4 hr
_______________
17kDa-

(B)
PARP
116
kDa-
-+ Precursor
85
kDa-
 -+
Cleaved product -+
Cleaved product
Fig. 3. Concentration- and
time-dependent caspase-3 activation and PARP cleavage
in sanguinarine-treated MDR HEN-16-2/CDDP cells.
Western blot analysis is shown for caspase-3 (A) and
PARP (B) using 10 ug protein/lane from cells
treated for 0.5 or 4 hr with sanguinarine at 0
uM (lanes I and 7), 1.06 uM (lanes 2 and
8), 2.12 uM (lanes 3 and 9), 4.24 uM
(lanes 4 and 10), 8.48 uM (lanes 5 and II), and
16.96 uM (lanes 6 and 12).
3.3. Induction of caspase-3
activation in apoptosis but
not oncotic cell death in both MDR HEN- I 6-2/CDDP and
drug-sensitive HEN-16-2 cells by
sanguinarine
To study the molecular
mechanism by which sanguinarine induces cell
morphologic changes, sanguinarine-treated cells were
examined for the proteolytic activation of caspase-3, a
downstream effector in apoptosis pathways. Sanguinarine
induced time- and concentration-dependent activation of
caspase-3 in MDR HEN-16-2/CDDP cells, as observed by
western blotting (Fig. 3A). Treatment for 0.5 hr with
0-16.96 uM sanguinarine did not cause detectable
proteolytic activation of caspase-3; a 4-hr treatment
with 2.12 and 4.24 uM sanguinarine, but no other
concentration from 0 to 16.96 uM, induced
cleavage of procaspase-3 to the activated 17 kDa
caspase-3 fragment. These results are consistent with a
previous demonstration that apoptosis requires
caspase-3 activation [37].
PARP is a critical cellular substrate for
proteolysis by activated caspase-3 [38]. Therefore, we
also studied whether the activation of caspase-3 by
sanguinarine may lead to increased cleavage of PARP. In
a time- and concentration-dependent analysis of PARP
cleavage that parallels the one for caspase-3
activation, cleaved PARP fragments were found at only 4
hr in 2.12 and 4.24 uM sanguinarine-treated MDR
cells (Fig. 3B) and drug-sensitive cells (Table 1). For
several other cervical cell lines, including HEN-16-2T,
similar sanguinarine concentration- and time-dependent
caspase-3 and PARP results were observed indicating
apoptosis; both results were absent in BCD/oncosis
(Table 1). Overall, these results suggest that
sanguinarine may be equally effective against MDR and
drug-sensitive human cervical cells, and act despite
MDR through bimodal apoptosis and BCD/oncosis pathways
having mechanisms that involve differential
morphologies and caspase-3 activation status.
4. Discussion
We established the in
vitro MDR cervical cell system used in this report
by treating HPV 16-immortalized human endocervical
HEN-16-2 cells with cisplatin [29]. Cell viability was
significantly higher in the MDR HEN-16- 2/CDDP cells
than in the parental cells after treatment with
cisplatin, actinomycin D, doxorubicin, etoposide,
paclitaxel, 5-fluorouracil, staurosporine, heat shock,
or UV radiation [29,39]. However, this study found no
significant difference in the effect of sanguinarine on
cell viability or clonogenic survival between the MDR
HEN-16-2/CDDP cells and their parental drug-sensitive
HEN-16-2 cells. Similarly, there was no significant
difference in cell death induced by sanguinarine
between CEM- VLB leukemia cells in which P-glycoprotein
(P-gp) mediates MDR and their wild-type drug-sensitive
counterpart CEM- T4 cells, which are P-gp-negative
(Table I). Importantly, sanguinarine has been found to
be selectively less toxic to normal cells [7]. Thus,
sanguinarine may be regarded as a potential therapeutic
agent even for MDR of certain types of transformed
cells, which are represented by HEN-16-2/CDDP cells
[29].
Proteolytic
activation of effector caspases, especially caspase-3,
is one of the key events in apoptosis [38,40]. The
results presented here show that sanguinarine induced
both apoptosis and BCD/oncosis in cervical MDR HEN-
16-2/CDDP cells and drug-sensitive HEN-16-2 cells.
Lower concentrations of sanguinarine induced apoptosis,
displayed by cell surface blebbing (Fig. 2C and D) and
caspase-3 activation, the latter confirmed by induction
of proteolytic cleavage of the caspase-3 substrate PARP
(Fig. 3). Higher concentrations of sanguinarine induced
cell death characterized by blistering, an oncotic late
cell death observed previously [41], and the absence of
caspase-3 activation (Figs. 2E and F and 3).
Bimodal cell death was also found to
be induced by sanguinarine in K562 erythroleukemia
cells [42], JMl pre-B lymphoblastic cells [42], MDR
CEM- VLB leukemia, and their wild-type counterpart
CEM-T4 cells (Table 1). Ukrain, an alkaloid derived
from the same plant family as sanguinarine, has been
reported to also induce apoptotic and blister forms
during K562leukemia cell death [21], and in MDR
HEN-16-2/CDDP and drug-sensitive HEN-16-2 cells (Table
1). Electronic transmission microscopy of K562 cells
showed that sanguinarine-induced apoptosis produced
classic morphologic changes, including the formation of
apoptotic bodies containing organelles and chromatin
condensation [42], whereas sanguinarine-induced oncosis
produced blisters that were devoid of organelles and
displayed patchy chromatin condensation [42].
BCD/oncosis is a form of cell death
that is distinct from apoptosis [43], whereas necrosis
refers to the intracellular degradative reactions
occurring after cell death by any mechanism, including
apoptosis [44]. Oncosis has been documented in many
studies [45-50]. The molecular and biochemical
mechanisms underlying oncosis are still unclear.
Oncosis was believed to result from a failure of plasma
membrane ionic pumps and decreased levels of cellular
ATP [51]. However, cell surface proteins, including
phospholipase A2 and Porimin, have been documented to
be involved in the process of cell membrane injury and
membrane structural changes [49,50,52]. Sanguinarine-
induced cell death pathways may be initiated that, if
not blocked, lead to caspase-3 activation, cleavage of
PARP and other caspase-3 substrates, and consequent
apoptotic cell death. If these pathways are blocked,
then other downstream or parallel steps of a pathway
may lead to caspase-independent oncosis [53-58]. Future
studies on the sanguinarine-activated cellular factors
involved in cell death pathways may provide a greater
understanding of the bimodal cell death pathways.
In summary, the data in this report
indicate that sanguinarine induces
concentration-dependent apoptosis with caspase-3
activation and BCD/oncosis without caspase-3
activation. The ability of this drug to induce bimodal
cell death modes at comparable efficiencies in MDR and
drug- sensitive human cervical and leukemia cells
indicates that sanguinarine was effective against MDR
in this in vitro system, and that there may be
two sanguinarine-induced cell death mechanisms.
Acknowledgments
We thank Mr. G. Chemenko,
Ms. Y. Hao, and Ms. L. Lee for excellent technical
assistance. The work was supported by a National Cancer
Institute of Canada grant (2734 to A.P.) with funds
from the Canadian Cancer Society; Medical Research
Council of Canada grants (MT -9782 and MT- 10140
to A.P. and MT-13178 to A.L.), and a Canadian
Institutes of Health Research (CIHR) grant (ROP-40859
to A.P.).
References
[I] Shamma M, Guinaudeau H.
Aporphinoid alkaloids. Nat Prod Rep 319
1986;3:345-51.
[2] Kuftinec MM, Mueller-Joseph LJ, Kopczyk
RA. Sanguinaria tooth- 321 paste and oral rinse regimen
clinical efficacy in short- and long-tenD 322 trials. J
Can Dent Assoc 1990;56:31-3. 323
[3] Laster LL, Lobene RR. New perspectives
on Sanguinaria clinicals: 324 individual toothpaste and
oral rinse testing. J Can Dent Assoc 1990; 325
56:19-30. 326
[4] Godowski KC, Wolff ED, Thompson DM, Housley
CJ, Polson AM, 327 Dunn RL, Duke SP, Stoller NH,
Southard GL. Whole mouth 328 microbiota effects
following subgingival delivery of sanguinarium. J 329
PeriodontoI1995;66:870-7. 330
[5] Colombo ML, Bosisio E. Pharmacological
activities of Chelidonium 331 majus L.
(Papaveraceae). Pharmacol Res 1996;33:127-34. 332
[6] Faddeeva MD, Beliaeva TN. Sanguinarine and
ellipticine cytotoxic 333 alkaloids isolated from
well-known antitumor plants. Intracellular 334 targets
of their action. Tsitologiia 1997;39:181-208. 335
[7] Ahmad N, Gupta S, Husain MM, Heiskanen KM,
Mukhtar H. 336 Differential antiproliferative and
apoptotic response of sanguinarine 337 for cancer cells
versus normal cells. Clin Cancer Res 2000;6:1524-8.
338
[8] Walterova D, Ulrichova J, Valka I, Vicar J,
Vavreckova C, Taborska 339 E, Harjrader RJ, Meyer DL,
Cerna H, Simanek V. Benzo[c]phenan-
thridine alkaloids
sanguinarine and chelerythrine: biological activ- 341
ities and dental care applications. Acta Univ Palacki
Olomuc Fac 342 Med 1995;139:7-16. 343
[9] Nandi R, Maiti M. Binding of sanguinarine to
deoxyribonucleic acids 344 of differing base
composition. Biochem PharmacoI1985;34:321-4. 345
[10] Saran A, Srivastava S, Coutinho E, Maiti M. IH NMR
investigation 346 of the interaction of berberine and
sanguinarine with DNA. Indian J 347 Biochem Biophys
1995;32:74-7. 348
[II] Wolff J, Knipling L. Antimicrotubule properties of
benzophenan- 349 thridine alkaloids. Biochemistry
1993;32:13334-9. 350
[12] Babich H, Zuckerbraun HL, Barber IB, Babich SB,
Borenfreund E. 351 Cytotoxicity of sanguinarine
chloride to cultured human cells from 352 oral tissue.
Pharmacol ToxicoI1996;78:397-403. 353
[13] Schmeller T, Latz-Bruning B, Wink M. Biochemical
activities of 354 berberine, palmatine and sanguinarine
mediating chemical defence 355 against microorganisms
and herbivores. Phytochemistry 1997;44:257- 356 66.
357
[14] Das M, Khanna SK. Clinicoepidemiological,
toxicological, and safety 358 evaluation studies on
argemone oil. Crit Rev Toxicol 1997;27:273- 359 97.
360
[15] Wang BH, Lu ZX, Polya GM. Inhibition of eukaryote
protein kinases 361 by isoquinoline and oxazine
alkaloids. Planta Med 1997;63:494-8. 362
[16] Chaturvedi MM, Kumar A, Damay BG, Chainy GB,
Agarwal S, 363 Aggarwal BB. Sanguinarine
(pseudochelerythrine) is a potent inhibitor 364 of
NF-KB
activation, IKB-(X phosphorylation, and degradation. J
Bioi Chem 1997;272:30129-34.
[17] OITenius S. Apoptosis: molecular mechanisms
and implications for human disease. J Intern Med
1995;237:529-36.
[18] Thompson CB. Apoptosis in the pathogenesis
and treatment of disease. Science 1995 ;267:
1456-62.
[19] White E. Life, death, and the pursuit of
apoptosis. Genes Dev 1996;10:1-15.
[20] Liepins A. Morphological, physiological and
biochemical parameters associated with cell injury: a
review. Immunopharmacol Immunotox-
icoI1989;11:539-58.
[21] Liepins A, Nowicky JW, Bustamante JO, Lam E.
Induction of bimodal programmed cell death in malignant
cells by the derivative Ukrain (NSC-631570). Drugs Exp
Clin Res 1996;22:73-9.
[22] Smith CA, Farrah T, Goodwin RG. The TNF
receptor superfamily of cellular and viral proteins:
activation, costimulation, and death. Cell
1994;76:959-62.
[23] Tartaglia LA, Rothe M, Hu YF, Goeddel DV.
Tumor necrosis factor's cytotoxic activity is signaled
by the p55 TNF receptor. Cell 1993;73:213-6.
[24] Trauth BC, KIas C, Peters AM, Matzku S,
Moller P, Falk W, Debatin KM, Krammer PH. Monoclonal
antibody-mediated tumor regression by induction of
apoptosis. Science 1989;245:301-5.
[25] Houghton JA. Apoptosis and drug response. CUlT
Opin Oncol 1999;11:475-81.
[26] Pisani P, Parkin DM, Bray F, Ferlay J. Estimates
of the worldwide mortality from 25 cancers in 1990. Int
J Cancer 1999;83:18-29.
[27] Parkin DM, Pisani P, Ferlay J. Estimates of the
worldwide incidence of 25 major cancers in 1990. Int J
Cancer 1999;80:827-41.
[28] Ma L, Krishnamachary N, Center MS. Phosphorylation
of the multidrug resistance associated protein gene
encoded protein P190. Biochemistry 1995;34:3338-43.
[29] Ding Z, Yang X, Chernenko G, Tang SC, Pater A.
Human papillomavirus type 16-immortalized endocervical
cells selected for resistance to cisplatin are
malignantly transformed and have a multidrugresistance
phenotype. Int J Cancer 2000;87:818-23.
[30] Tsutsumi K, Belaguli N, Qi S, Michalak TI,
Gulliver WP, Pater A, Pater MM. Human papillomavirus 16
DNA immortalizes two types of normal human epithelial
cells of the uterine cervix. Am J Pathol
1992;140:255-61.
[31] Yang X, Jin G, Nakao Y, Rahimtula M, Pater MM,
Pater A. Malignant transformation of HPV
16-immortalized human endocer- vical cells by cigarette
smoke condensate and characterization of multistage
carcinogenesis. Int J Cancer 1996;65:338-44.
[32] Yang X, Hao Y, Ding Z, Pater A. BAG-I promotes
apoptosis induced by N-(4-hydroxyphenyl)retinamide in
human cervical carcinoma cells. Exp Cell Res
2000;256:491-9.
[33] Schmid I, Uittenbogaart CH, Giorgi JV. Sensitive
method for measuring apoptosis and cell surface
phenotype in human thymo- cytes by flow cytometry.
Cytometry 1994;15:12-20.
[34] Vasey PA, Jones NA, Jenkins S, Dive C, Brown R.
Cisplatin, camptothecin, and taxol sensitivities of
cells with p53-associated multidrug resistance. Mol
Pharmacol 1996;50: 1536-40.
[35] Liepins A, Younghusband HB. A possible role for K+
channels in tumor cell injury. Membrane vesicle
shedding and nuclear DNA fragmentation. Exp Cell Res
1987;169:385-94.
[36] Yang X, Hao Y, Pater MM, Tang S-C, Pater A.
Enhanced expression of anti-apoptotic proteins in human
papillomavirus-immortalized and cigarette smoke
condensate-transformed human endocervical cells:
coITelation with resistance to apoptosis induced by DNA
damage. Mol Carcinog 1998;22:95-101.
[37] Janicke RU, Sprengart ML, Wati MR, Porter AG.
Caspase-3 is required for DNA fragmentation and
morphological changes associated with apoptosis. J Bioi
Chem 1998;273:9357-60.
[38] Cryns V, Yuan J. Proteases to die for. Genes Dev
1998;12:1551-70.
[39] Ding Z, Yang X, Pater A, Tang
SC. Resistance to apoptosis is 430 correlated with the
reduced caspase-3 activation and enhanced 431
expression of antiapoptotic proteins in human cervical
multidrug- 432 resistant cells. Biochem Biophys Res
Cornrnun 2000;270:415-20. 433
[40] Nunez 0, Benedict MA, Hu Y, Inohara N.
Caspases: the proteases of 434 the apoptotic pathway.
Oncogene 1998;17:3237-45. 435
[41] Collins JA, Schandi CA, Young KK, Vesely J,
Willingham MC. 436 Major DNA fragmentation is a late
event in apoptosis. J Histochem 437 Cytochem
1997;45:923-34. 438
[42] Weerasinghe P, Hallock S, Liepins A. Bax,
bcl-2, and NF-ICB 439 expression in sanguinarine
induced bimodal cell death. Exp Mol 440
PathoI2001;71:89-98. 441
[43] Trump BF, Berezesky IK, Chang SH, Phelps PC.
The pathways of 442 cell death: oncosis, apoptosis, and
necrosis. Toxicol Pathol 1997;25: 443 82-8. 444
[44] Majno 0, Joris I. Apoptosis, oncosis and
necrosis. An overview of 445 cell death. Am J
PathoI1995;146:3-15. 446
[45] Fernandez-Prada CM, Hoover DL, Tall BD,
Venkatesan MM. Human 447 monocyte-derived macrophages
infected with virulent Shigella 448
flexneri in vitro undergo a rapid cytolytic
event similar to oncosis 449 but not apoptosis. Infect
Irnrnun 1997;65:1486-96. 450
[46] Kuwashima Y. Cytomorphology of murine BI6
melanoma in vivo 451 after treatment with
cyclophosphamide: evidence of "oncotic" cell 452 death.
Anticancer Res 1996;16:2997-3000. 453
[47] Jonas D, Walev I, Berger T, Liebetrau M, Palmer M,
Bhakdi S. Novel 454 path to apoptosis: small
transmembrane pores created by staphylo- 455 coccal
alpha-toxin in T lymphocytes evoke internucleosomal DNA
456 degradation. Infect Irnrnun 1994;62:1304-12.
457
[48] Matsuoka S, Asano Y, Sano K, Kishimoto H,
Yamashita I, Yorifuji H, 458 Utsuyama M, Hirokawa K,
Tada T. A novel type of cell death of 459 lymphocytes
induced by a monoclonal antibody without participation
460 of complement. J Exp Med 1995;181:2007-15. 461
[49] CUmmings BS, McHowat J, Schnellrnann RO.
Phospholipase Azs in 462 cell injury and death. J
Pham)acol Exp Ther 2000;294:793-9. 463
[50] Ma F, Zhang C, Prasad KV, Freeman OJ, Schlossman
SF. Molecular 464 cloning of ~ a novel cell surface
receptor mediating oncotic 465
cell death. Proc Natl
Acad Sci USA 2001;98:9778-83. 466 [51] Eguchi Y,
Shimizu S, Tsujimoto Y. Intracellular ATP levels
467
determine cell death fate
by apoptosis or necrosis. Cancer Res 468
1997;57:1835-40.
[52] Sapirstein A, Bonventre IV. Specific physiological
roles of cytosolic 470 phospholipase Az as defined by
gene knockouts. Biochim Biophys 471 Acta
2000;1488:139-48. 472
[53] Zanke BW, Lee C, Arab S, Tannock IF. Death of
tumor cells after 473 intracellular acidification is
dependent on stress-activated protein 474 kinases
(SAPK/JNK) pathway activation and cannot be inhibited
by 475 Bcl-2 expression or interleukin 1/3-converting
enzyme inhibition. 476 Cancer Res 1998;58:2801-8.
477
[54] Henkels KM, Turchi II. Cisplatin-induced apoptosis
proceeds by 478 caspase-3-dependent and -independent
pathways in cisplatin-resistant 479 and -sensitive
human ovarian cancer cell lines. Cancer Res 480
1999;59:3077-83.
[55] Stefanis L, Park DS, Friedman WJ, Oreene LA.
Caspase-dependent 482 and -independent death of
camptothecin-treated embryonic cortical 483 neurons. J
Neurosci 1999;19:6235-47. 484
[56] Chi S, Kitanaka C, Noguchi K, Mochizuki T,
Nagashima Y, Shirouzu 485 M, Fujita H, Yoshida M, Chen
W, Asai A, Himeno M, Yokoyama S,
Kuchino Y. Oncogenic Ras
triggers cell suicide through the activation 487 of a
caspase-independent cell death program in human cancer
cells. 488 Oncogene 1999;18:2281-90. 489
[57] Kitanaka C, Kuchino Y. Caspase-independent
programmed cell death 490 with necrotic morphology.
Cell Death Differ 1999;6:508-15. 491
[58] Xue L, Fletcher OC, Tolkovsky AM. Autophagy is
activated by 492 apoptotic signalling in sympathetic
neurons: an alternative mechan- 493 ism of death
execution. Mol Cell Neurosci 1999;14:180-98. 494
. Corresponding author. Tel.:
+1-709-777-6488; fax: +1-709-777-7010. E-mail
address: apater@mun.ca (A. Pater).
Abbreviations: PCD, programm~d cell
death; COOP, cis-diamminedi- chloroplatinum (II),
cisplatin; MDR, multidrug resistance (or resistant);
HPV, human papillomavirus; CSC, cigarette smoke
condensate; PARP, poly(ADP-ribose) polymerase; BCD,
blister cell death/oncosis.
0006-2952/02/$ - see front
matter (j;;) 2002 Published by Elsevier Science
1m PII: SOO06-2952(02)00902-4 Z. Ding et
al.IBiochemical Phannacology 7194
(2002)
|